Chapter 4: States of Consciousness
4.1 Foundations of Consciousness
Learning Objectives
- Describe consciousness and circadian rhythms
- Explain blindsight and what it reveals about consciousness
- Explain disruptions in biological rhythms, including sleep debt
Why It Matters: States of Consciousness

Our lives involve regular, dramatic changes in the degree to which we are aware of our surroundings and our internal states. While awake, we feel alert and aware of the many important things going on around us. Our experiences change dramatically while we are in deep sleep and once again when we are dreaming. Sometimes, we seek to alter our awareness and experience by using psychoactive drugs; that is, drugs that alter the central nervous system and produce a change of consciousness or a deep meditative state. Consciousness is an awareness of external and internal stimuli. As discussed in the module on the biology of psychology, the brain activity during different phases of consciousness produces characteristic brain waves, which can be observed by electroencephalography (EEG) and other types of analysis.
This module will discuss states of consciousness with a particular emphasis on sleep. You’ll learn about the different stages of sleep, sleep disorders as well as the altered states of consciousness produced by psychoactive drugs, hypnosis, and meditation.
Introduction to Consciousness and Rhythms
What you’ll learn to do: describe consciousness and biological rhythms
Are you tired? Have you ever pulled an all-nighter? How did you feel the next day? Do you think your lack of sleep impacted your behavior? Chances are, you could answer that question with a resounding, “yes!”. Because psychologists are interested in mental processes and behavior, it’s essential to study consciousness, or our awareness, as humans. States of consciousness vary over the course of the day and throughout our lives, and sleep plays a major role in alertness levels. Important factors in daily changes in consciousness are biological rhythms, and, more specifically, the circadian rhythms generated by the suprachiasmatic nucleus. Typically, our biological clocks are aligned with our external environment, and light tends to be an important cue in setting this clock. When people travel across multiple time zones or work rotating shifts, they can experience disruptions of their circadian cycles that can lead to insomnia, sleepiness, and decreased alertness. If people go extended periods of time without sleep, they will accrue a sleep debt and potentially experience a number of adverse psychological and physiological consequences.
Consciousness and Biological Rhythms
Consciousness describes our awareness of internal and external stimuli. Awareness of internal stimuli includes feeling pain, hunger, thirst, sleepiness, and being aware of our thoughts and emotions. Awareness of external stimuli includes seeing the light from the sun, feeling the warmth of a room, and hearing the voice of a friend.
We experience different states of consciousness and different levels of awareness on a regular basis. We might even describe consciousness as a continuum that ranges from full awareness to a deep sleep. Sleep is a state marked by relatively low levels of physical activity and reduced sensory awareness that is distinct from periods of rest that occur during wakefulness. Wakefulness is characterized by high levels of sensory awareness, thought, and behavior. In between these extremes are states of consciousness related to daydreaming, intoxication as a result of alcohol or other drug use, meditative states, hypnotic states, and altered states of consciousness following sleep deprivation. We might also experience unconscious states of being via drug-induced anesthesia for medical purposes. Often, we are not completely aware of our surroundings, even when we are fully awake. For instance, have you ever daydreamed while driving home from work or school without really thinking about the drive itself? You were capable of engaging in the all of the complex tasks involved with operating a motor vehicle even though you were not aware of doing so. Many of these processes, like much of psychological behavior, are rooted in our biology.
Biological Rhythms
Biological rhythms are internal rhythms of biological activity. A woman’s menstrual cycle is an example of a biological rhythm—a recurring, cyclical pattern of bodily changes. One complete menstrual cycle takes about 28 days—a lunar month—but many biological cycles are much shorter. Biological rhythms such as the menstrual cycle are called infradian rhythms because they last longer than 24 hours, and others that last less than 24 hours are called ultradian rhythms. Changes in body temperature and alertness that fluctuate cyclically over a 24-hour period (Figure 1) are examples of a circadian rhythm. A circadian rhythm is a biological rhythm that takes place over a period of about 24 hours. Alertness is associated with higher body temperatures, and sleepiness with lower body temperatures.

Our sleep-wake cycle, which is linked to our environment’s natural light-dark cycle, is perhaps the most obvious example of a circadian rhythm, but we also have daily fluctuations in heart rate, blood pressure, blood sugar, and body temperature. Some circadian rhythms play a role in changes in our state of consciousness.
Research indicates that humans (as well as other animals and plants) have a biological clock, or an innate timing device, comprised of specific molecules (proteins) that interact in cells throughout the body. Biological clocks are found in nearly every tissue and organ. Researchers have identified similar genes in people, fruit flies, mice, fungi, and several other organisms that are responsible for making the clock’s components. In the brain, the hypothalamus, which lies above the pituitary gland, is a main center of homeostasis. Homeostasis is the tendency to maintain a balance, or optimal level, within a biological system. In people, the brain’s clock mechanism is located in an area of the hypothalamus known as the suprachiasmatic nucleus (SCN). The SCN is comprised of about 20,000 nerve cells. The axons of light-sensitive neurons in the retina provide information to the SCN based on the amount of light present, allowing this internal clock to be synchronized with the outside world (Klein, Moore, & Reppert, 1991; Welsh, Takahashi, & Kay, 2010) (Figure 2).

Problems with Circadian Rhythms
Generally, and for most people, our circadian cycles are aligned with the outside world. For example, most people sleep during the night and are awake during the day. One important regulator of sleep-wake cycles is the hormone melatonin. The pineal gland, an endocrine structure located inside the brain that releases melatonin, is thought to be involved in the regulation of various biological rhythms and of the immune system during sleep (Hardeland, Pandi-Perumal, & Cardinali, 2006). Melatonin release is stimulated by darkness and inhibited by light. People rely on zeitgebers, or external cues, such as light, atmospheric conditions, temperature, and social interactions, to set the appropriate biological clock.
There are individual differences with regards to our sleep-wake cycle. For instance, some people would say they are morning people, while others would consider themselves to be night owls. These individual differences in circadian patterns of activity are known as a person’s chronotype. A person’s individual chronotype may show that a person has a greater propensity to sleep earlier and wake up earlier (a morning lark), or to stay up late and sleep in (a night owl). Morning larks and night owls differ with regard to sleep regulation (Taillard, Philip, Coste, Sagaspe, & Bioulac, 2003). Sleep regulation refers to the brain’s control of switching between sleep and wakefulness as well as coordinating this cycle with the outside world.
Link to Learning
Watch this brief video describing circadian rhythms and how they affect sleep.
Try It
Think It Over
Psych in Real Life: Consciousness and Blindsight
If you have already studied about the brain (in the Biopsychology module) then the picture below of the four major lobes of the cerebral cortex should look familiar. Click on the part of the brain that is most heavily involved in vision.
Blindsight
What do you think would happen if your occipital lobes were damaged? Back in the 1970s, most scientists and physicians would have said, “you would become blind.” It turns out that the answer is more complicated than that.
When he was 8-years old, Graham Young from Oxford, England, was injured in a bicycle accident. Afterwards, he reported that parts of his vision were gone. He told his doctors that he could no longer see anything to the right of his center of vision with either his left or right eye. The left side of his visual world in both eyes was normal. Although he says that he would sometimes walk into objects to his right because he couldn’t see them, when tested fifteen years later, an optician discovered that Mr. Young seemed to respond to visual movements in his “blind” area.
Graham Young was put into contact with Psychologists Larry Weiskrantz and Elizabeth Warrington, who had worked previously with a person (known as DB) who seemed to have a similar ability to see despite blindness. DB could report shapes and colors, movement and the orientation of objects despite claiming that he could see nothing. He said that he was guessing, but he was usually right about colors and shapes and other characteristics of the objects.
Before we go on, please take a moment to theorize about what might be going on with Graham Young and DB.
People with blindsight have been tested for their ability to detect color differences, brightness changes, the ability to discriminate between various shapes, as well as tracking movement. Critically, people with blindsight have the conscious experience of blindness, often feeling like they are guessing despite their high level of accuracy.
Blindsight in Action
Here is a brief video of the man who experiences complete blindness because his visual cortex in both hemispheres has been damaged. The researchers (including Dr. Weiskrantz, mentioned above) set up an obstacle course for the man (whose face is blurred to protect his privacy). Watch how well he moves through the objects without help. The man behind him is just there as a safety precaution.
How can blindsight happen?
Your conscious experience of the world around you, of the choices and decisions you make, and of the emotions and attitudes that motivate you are not the totality of your mental activity or of your brain’s processing of information. Many, perhaps most, psychologists believe that consciousness is only a small part of your total cognitive activity.[1]
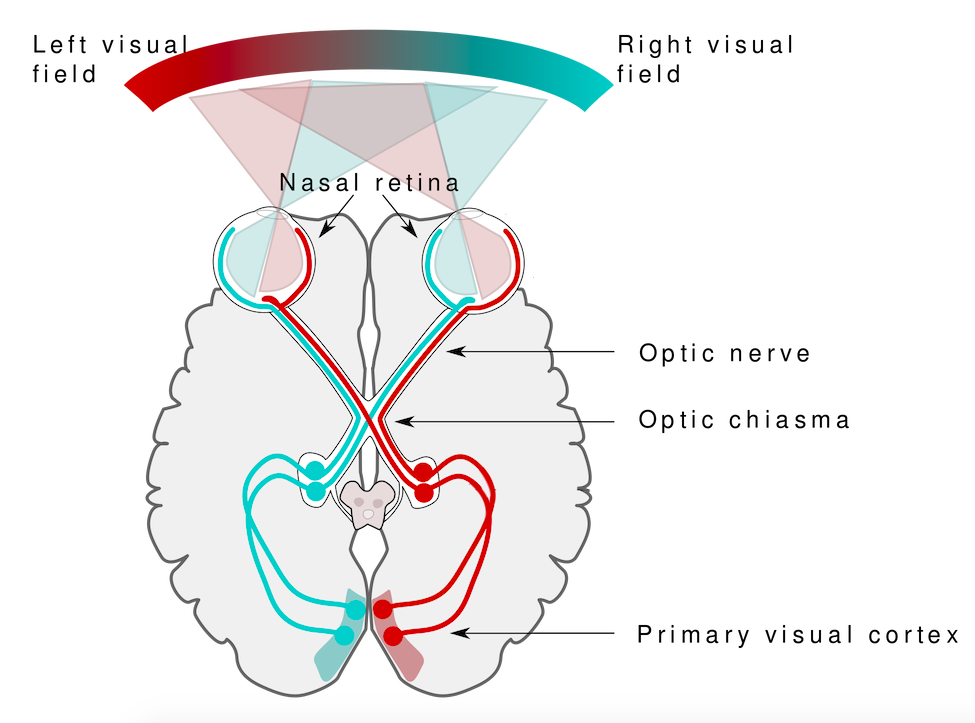
A person is considered to be blind if he or she has no conscious experience of the visual world. This conscious experience is based on the flow of information from the eyes through the thalamus in the middle of the brain to the primary visual cortex in the occipital lobe at the back of the brain. If the primary visual cortex is damaged or fails to receive input due to disruption of visual pathway, then the person will not “see” the objects and events that we normally associate with vision.

Blindsight occurs because the visual system has a primary pathway (retina to thalamus to primary visual cortex), but it also has secondary pathways (retina to thalamus to other brain areas). These “other brain areas” include parts of the frontal lobe that guide eye movements, parts of the midbrain that help guide visual attention, and parts of the occipital lobe that process features of the visual perception, including shape, movement, and color.[2]
The existence of visual processing areas for isolated features of vision and the fact that these areas get some direct visual information (i.e, input that does not first go to the primary visual cortex) means that it is possible for a person to respond accurately to questions about color or motion or shape without consciously “seeing” the objects that have color or shape or are moving.
It is important to remember that YOU have these same “unconscious” pathways in your visual system. That means your conscious experience of the visual world may not include all of the visual information you are processing. In other words, you may “know” more than you “see”.
Blindsight is not the only condition that involves unconscious or low-consciousness processing. Other neurological syndromes that have an unconscious element include amnesia, hemispatial neglect, dyslexia, aphasia, and various agnosias.[3]
Creating Blindsight in the Laboratory
Wouldn’t it be great if we could produce blindsight in the laboratory, in order to better understand visual processing and conscious experience? Maybe with college student volunteers as our subjects? Crazy idea?

It turns out, researchers have already done it. Using precisely aimed magnetic pulses, researchers can temporarily disrupt specific areas of the primary visual cortex—the area responsible for conscious vision—without injury. This “blindness” lasts only a fraction of a second, after which vision returns to normal. Would you volunteer to be a participant?
Let’s look at how this works.
TMS: Transcranial Magnetic Stimulation
Transcranial magnetic stimulation (TMS) is a procedure used to stimulate neurons in the brain. A device referred to as a “wand” contains an electric coil that generates a magnetic field that in turn creates a small electric current in the brain.[4] The electric current induces neurons (brain cells) to produce neural signals called action potentials. When action potentials are produced in normal brain processes, they allow neurons to communicate with one another. However, when action potentials are induced by an outside force—here by the TMS wand—they are meaningless and temporarily interfere with communication between neurons. If only a single pulse of electromagnetic energy is produced, then the disruption of the neurons in the targeted region lasts only a fraction of a second. Multiple pulses, called repetitive TMS (rTMS), can produce longer lasting effects. In fact, rTMS is now used by therapists as a treatment for depression and neuropathic pain.
The TMS pulse can be aimed very precisely at a small area of the brain. When the target is the primary visual cortex in the occipital lobe, the TMS pulse can be focused to interfere with neural communication in a tiny region of the of the visual field—so small and occurring for such a short time that you would not even notice. However brief the duration or tiny the affected area, the person receiving the TMS pulse is temporary blind in a small part of the visual field.
Laboratory Research on Unconscious Visual Processing
Dr. Tony Ro is a professor of psychology at the City University of New York. He started studying the connection between consciousness and brain processing more than 20 years ago, and he was one of the earliest researchers to apply TMS technology to the study of visual perception.
In one study, Dr. Ro and graduate students Jennifer Boyer and Stephenie Harrison used TMS technology to see if normal people could process features of visual stimuli without conscious awareness of those stimuli. In other words, they wanted to know if they could they create temporary blindsight in normal subjects in a laboratory.
Remember that blindsight involves unconscious awareness of “features” of objects and events, such as the shape of an object or the direction of its movement. This study focused on two visual features: orientation and color. You and I see orientation (horizontal or vertical) or color (red or green) as part of the experience of some object. A line is horizontal. A box is red. For a person with blindsight, “horizontal” is experienced without any shape associated with it. “Red” is experienced without awareness of the thing that is red. This is the blindsight condition that Dr. Ro and his colleagues wanted to reproduce in the laboratory with the help of volunteer subjects.
Let’s walk through the experiment to understand how it was designed and conducted.
Experiment 1: Unconscious Detection of Orientation
SETUP: The TMS wand was precisely adjusted so the TMS pulse was aimed at the back of the brain (primary visual cortex in the occipital lobes) affecting a very small area of the visual field. For example, imagine the gray box below as a computer screen. The plus sign in the middle is a fixation point. You (the participant in the study) fixate your eyes on this plus sign and hold them there during each trial. The TMS pulse is adjusted to your individual brain so that the area shown as a blue circle (used here only for explanation purposes) is momentarily “blind” when the pulse is active. This is a painstaking process that involves fine calibration of the wand based on feedback from the participant about what he or she can see when different targets are shown on the screen.

TESTING: In one of Dr. Ro’s experiments, participants had to guess the orientation of a line, sometimes when they were temporarily blinded (in a tiny area of the visual cortex) by a TMS pulse. The study consisted of a series of trials. On each trial, either a horizontal or a vertical line was flashed for a fraction of a second on the computer screen in front of the participant. On some of these trials, a TMS pulse disrupted the neurons in the visual cortex. On other trials, there was no TMS pulse. The no-pulse trials served as a kind of control condition.
Click on the slideshow below to see the steps in the vertical line condition. You can use the arrows at the bottom to navigate through the slides.
RESULTS: By chance, if you have to choose between two equally likely options (horizontal or vertical), you would be correct about 50% of the time. On the trials when the subjects reported that they did not “see” anything at all, they correctly guessed the orientation of the line 75% of the time, performance that is significantly better than chance. There was also a strong positive correlation (r = +0.93) between accuracy and confidence: the more confident the subject in his or her guess, the more likely it was that the guess was correct. Keep in mind that, in all of these cases, the subjects started by saying that they saw nothing. That was about 60% of the trials. On the other 40% of trials, the subjects reported seeing something, even if it was a slight blur, and these trials did not count. Not surprisingly, accuracy was near perfect when subjects were conscious of seeing the bar and its orientation.
Variations of the Experiment
A second study using the color of a circle rather than the orientation of a bar was reported in the same paper. Otherwise, the procedures were the same as in the first experiment and the results consistent with the results for the bar orientation experiment.
Testing Blindsight with TMS
Here is a video about a similar experiment conducted by Dr. Ro and his colleagues. The experiment in the video involves detecting yet another feature of objects: their shape. The basic procedures and results are similar to the ones you have just read.
Conclusions from the Research
The experimenters succeeded in producing the experience of blindness using the TMS apparatus, and they also succeeded in producing evidence for unconscious processing of features of the visual experience in normal (college student) volunteers. These results, when put together with the experiences of people with neurological damage, strengthen the case for the theory that some of our visual perception of the world takes place outside of our awareness. The college students have shown that this unconscious processing is not the result of brain damage, but rather is part of our normal perception of the world.
Some Final Words
This module has been about consciousness. It is common to assume that everything we know about the world around us and about our own thoughts and internal experiences must go through the doorway of our conscious mind. Evidence from blindsight is just one of several lines of research that shows that we process more information than we are aware of. Learning just how much this unconscious information can influence our thoughts and actions, our preferences and beliefs, is an important challenge for the rising generation of scientists.
When Biological Clocks Get Disrupted
Disruptions of Normal Sleep
Whether lark, owl, or somewhere in between, there are situations in which a person’s circadian clock gets out of synchrony with the external environment. One way that this happens involves traveling across multiple time zones. When we do this, we often experience jet lag. Jet lag is a collection of symptoms that results from the mismatch between our internal circadian cycles and our environment. These symptoms include fatigue, sluggishness, irritability, and insomnia (i.e., a consistent difficulty in falling or staying asleep for at least three nights a week over a month’s time) (Roth, 2007).
Individuals who do rotating shift work are also likely to experience disruptions in circadian cycles. Rotating shift work refers to a work schedule that changes from early to late on a daily or weekly basis. For example, a person may work from 7:00 a.m. to 3:00 p.m. on Monday, 3:00 a.m. to 11:00 a.m. on Tuesday, and 11:00 a.m. to 7:00 p.m. on Wednesday. In such instances, the individual’s schedule changes so frequently that it becomes difficult for a normal circadian rhythm to be maintained. This often results in sleeping problems, and it can lead to signs of depression and anxiety. These kinds of schedules are common for individuals working in health care professions and service industries, and they are associated with persistent feelings of exhaustion and agitation that can make someone more prone to making mistakes on the job (Gold et al., 1992; Presser, 1995).
Rotating shift work has pervasive effects on the lives and experiences of individuals engaged in that kind of work, which is clearly illustrated in stories reported in a qualitative study that researched the experiences of middle-aged nurses who worked rotating shifts (West, Boughton & Byrnes, 2009). Several of the nurses interviewed commented that their work schedules affected their relationships with their family. One of the nurses said,
If you’ve had a partner who does work regular job 9 to 5 office hours . . . the ability to spend time, good time with them when you’re not feeling absolutely exhausted . . . that would be one of the problems that I’ve encountered. (West et al., 2009, p. 114)
While disruptions in circadian rhythms can have negative consequences, there are things we can do to help us realign our biological clocks with the external environment. Some of these approaches, such as using a bright light as shown in Figure 1, have been shown to alleviate some of the problems experienced by individuals suffering from jet lag or from the consequences of rotating shift work. Because the biological clock is driven by light, exposure to bright light during working shifts and dark exposure when not working can help combat insomnia and symptoms of anxiety and depression (Huang, Tsai, Chen, & Hsu, 2013).

Insufficient Sleep
When people have difficulty getting sleep due to their work or the demands of day-to-day life, they accumulate a sleep debt. A person with a sleep debt does not get sufficient sleep on a chronic basis. The consequences of sleep debt include decreased levels of alertness and mental efficiency. Interestingly, since the advent of electric light, the amount of sleep that people get has declined. While we certainly welcome the convenience of having the darkness lit up, we also suffer the consequences of reduced amounts of sleep because we are more active during the nighttime hours than our ancestors were. As a result, many of us sleep less than 7–8 hours a night and accrue a sleep debt. While there is tremendous variation in any given individual’s sleep needs, the National Sleep Foundation (n.d.) cites research to estimate that newborns require the most sleep (between 12 and 18 hours a night) and that this amount declines to just 7–9 hours by the time we are adults.
If you lie down to take a nap and fall asleep very easily, chances are you may have sleep debt. Given that college students are notorious for suffering from significant sleep debt (Hicks, Fernandez, & Pelligrini, 2001; Hicks, Johnson, & Pelligrini, 1992; Miller, Shattuck, & Matsangas, 2010), chances are you and your classmates deal with sleep debt-related issues on a regular basis. The table below shows recommended amounts of sleep at different ages.
| Age | Nightly Sleep Needs |
|---|---|
| 0–3 months | 12–18 hours |
| 3 months–1 year | 14–15 hours |
| 1–3 years | 12–14 hours |
| 3–5 years | 11–13 hours |
| 5–10 years | 10–11 hours |
| 10–18 years | 8–10 hours |
| 18 and older | 7–9 hours |
Sleep debt and sleep deprivation have significant negative psychological and physiological consequences. As mentioned earlier, lack of sleep can result in decreased mental alertness and cognitive function. In addition, sleep deprivation often results in depression-like symptoms. These effects can occur as a function of accumulated sleep debt or in response to more acute periods of sleep deprivation. It may surprise you to know that sleep deprivation is associated with obesity, increased blood pressure, increased levels of stress hormones, and reduced immune functioning (Banks & Dinges, 2007). Furthermore, individuals suffering from sleep deprivation can also put themselves and others at risk when they put themselves behind the wheel of a car or work with dangerous machinery. Some research suggests that sleep deprivation affects cognitive and motor function as much as, if not more than, alcohol intoxication (Williamson & Feyer, 2000).

The amount of sleep we get varies across the lifespan. When we are very young, we spend up to 16 hours a day sleeping. As we grow older, we sleep less. In fact, a meta-analysis, which is a study that combines the results of many related studies, conducted within the last decade indicates that by the time we are 65 years old, we average fewer than 7 hours of sleep per day (Ohayon, Carskadon, Guilleminault, & Vitiello, 2004). As the amount of time we sleep varies over our lifespan, presumably the sleep debt would adjust accordingly.
Try It
Think It Over
What do you do to adjust to the differences in your daily schedule throughout the week? Are you running a sleep debt when daylight saving time begins or ends?
Module References (Click to expand)
Aggarwal, S. K., Carter, G. T., Sullivan, M. D., ZumBrunnen, C., Morrill, R., & Mayer, J. D. (2009). Medicinal use of cannabis in the United States: Historical perspectives, current trends, and future directions. Journal of Opioid Management, 5, 153–168.
Alhola, P. & Polo-Kantola, P. (2007). Sleep Deprivation: Impact on cognitive performance. Neuropsychiatric Disease and Treatment, 3, 553–557.
Alladin, A. (2012). Cognitive hypnotherapy for major depressive disorder. The American Journal of Clinical Hypnosis, 54, 275–293.
American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Arlington, VA: Author.
Aquina, C. T., Marques-Baptista, A., Bridgeman, P., & Merlin, M. A. (2009). Oxycontin abuse and overdose. Postgraduate Medicine, 121, 163–167.
Arnulf, I. (2012). REM sleep behavior disorder: Motor manifestations and pathophysiology. Movement Disorders, 27, 677–689.
Augustinova, M., & Ferrand, L. (2012). Suggestion does not de-automatize word reading: Evidence from the semantically based Stroop task. Psychonomic Bulletin & Review, 19, 521–527.
Banks, S., & Dinges, D. F. (2007). Behavioral and physiological consequences of sleep restriction. Journal of Clinical Sleep Medicine, 3, 519–528.
Bartke, A., Sun, L. Y., & Longo, V. (2013). Somatotropic signaling: Trade-offs between growth, reproductive development, and longevity. Physiological Reviews, 93, 571–598.
Berkowitz, C. D. (2012). Sudden infant death syndrome, sudden unexpected infant death, and apparent life-threatening events. Advances in Pediatrics, 59, 183–208.
Berry, R. B., Kryger, M. H., & Massie, C. A. (2011). A novel nasal excitatory positive airway pressure (EPAP) device for the treatment of obstructive sleep apnea: A randomized controlled trial. Sleep, 34, 479–485.
Bixler, E. O., Kales, A., Soldatos, C. R., Kales, J. D., & Healey, S. (1979). Prevalence of sleep disorders in the Los Angeles metropolitan area. American Journal of Psychiatry, 136, 1257–1262.
Bostwick, J. M. (2012). Blurred boundaries: The therapeutics and politics of medical marijuana. Mayo Clinic Proceedings, 87, 172–186.
Brook, R. D., Appel, L. J., Rubenfire, M., Ogedegbe, G., Bisognano, J. D., Elliott, W. K., . . . Rajagopalan, S. (2013). Beyond medications and diet: Alternative approaches to lowering blood pressure: A scientific statement from the American Heart Association. Hypertension, 61, 1360–1383.
Broughton, R., Billings, R., Cartwright, R., Doucette, D., Edmeads, J., Edwardh, M., . . . Turrell, G. (1994). Homicidal somnambulism: A case report. Sleep, 17, 253–264.
Brown, L. K. (2012). Can sleep deprivation studies explain why human adults sleep? Current Opinion in Pulmonary Medicine, 18, 541–545.
Burgess, C. R., & Scammell, T. E. (2012). Narcolepsy: Neural mechanisms of sleepiness and cataplexy. Journal of Neuroscience, 32, 12305–12311.
Cai, D. J., Mednick, S. A., Harrison, E. M., Kanady, J. C., & Mednick, S. C. (2009). REM, not incubation, improves creativity by priming associative networks. Proceedings of the National Academy of Sciences, USA, 106, 10130–10134.
Caldwell, K., Harrison, M., Adams, M., Quin, R. H., & Greeson, J. (2010). Developing mindfulness in college students through movement based courses: Effects on self-regulatory self-efficacy, mood, stress, and sleep quality. Journal of American College Health, 58, 433–442.
Capellini, I., Barton, R. A., McNamara, P., Preston, B. T., & Nunn, C. L. (2008). Phylogenetic analysis of the ecology and evolution of mammalian sleep. Evolution, 62, 1764–1776.
Cartwright, R. (2004). Sleepwalking violence: A sleep disorder, a legal dilemma, and a psychological challenge. American Journal of Psychiatry, 161, 1149–1158.
Cartwright, R., Agargun, M. Y., Kirkby, J., & Friedman, J. K. (2006). Relation of dreams to waking concerns. Psychiatry Research, 141, 261–270.
Casati, A., Sedefov, R., & Pfeiffer-Gerschel, T. (2012). Misuse of medications in the European Union: A systematic review of the literature. European Addiction Research, 18, 228–245.
Chen, K. W., Berger, C. C., Manheimer, E., Forde, D., Magidson, J., Dachman, L., & Lejuez, C. W. (2013). Meditative therapies for reducing anxiety: A systematic review and meta-analysis of randomized controlled trials. Depression and Anxiety, 29, 545–562.
Chokroverty, S. (2010). Overview of sleep & sleep disorders. Indian Journal of Medical Research, 131, 126–140.
Christensen, A., Bentley, G. E., Cabrera, R., Ortega, H. H., Perfito, N., Wu, T. J., & Micevych, P. (2012). Hormonal regulation of female reproduction. Hormone and Metabolic Research, 44, 587–591.
CNN. (1999, June 25). ‘Sleepwalker’ convicted of murder. Retrieved from http://www.cnn.com/US/9906/25/sleepwalker.01/
Cropley, M., Theadom, A., Pravettoni, G., & Webb, G. (2008). The effectiveness of smoking cessation interventions prior to surgery: A systematic review. Nicotine and Tobacco Research, 10, 407–412.
De la Herrán-Arita, A. K., & Drucker-Colín, R. (2012). Models for narcolepsy with cataplexy drug discovery. Expert Opinion on Drug Discovery, 7, 155–164.
Del Casale, A., Ferracuti, S., Rapinesi, C., Serata, D., Sani, G., Savoja, V., . . . Girardi, P. (2012). Neurocognition under hypnosis: Findings from recent functional neuroimaging studies. International Journal of Clinical and Experimental Hypnosis, 60, 286–317.
Elkins, G., Johnson, A., & Fisher, W. (2012). Cognitive hypnotherapy for pain management. The American Journal of Clinical Hypnosis, 54, 294–310.
Ellenbogen, J. M., Hu, P. T., Payne, J. D., Titone, D., & Walker, M. P. (2007). Human relational memory requires time and sleep. Proceedings of the National Academy of Sciences, USA, 104, 7723–7728.
Fell, J., Axmacher, N., & Haupt, S. (2010). From alpha to gamma: Electrophysiological correlates meditation-related states of consciousness. Medical Hypotheses, 75, 218–224.
Fenn, K. M., Nusbaum, H. C., & Margoliash, D. (2003). Consolidation during sleep of perceptual learning of spoken language. Nature, 425, 614–616.
Ferini-Strambi, L. (2011). Does idiopathic REM sleep behavior disorder (iRBD) really exist? What are the potential markers of neurodegeneration in iRBD [Supplemental material]? Sleep Medicine, 12(2 Suppl.), S43–S49.
Fiorentini, A., Volonteri, L.S., Dragogna, F., Rovera, C., Maffini, M., Mauri, M. C., & Altamura, C. A. (2011). Substance-induced psychoses: A critical review of the literature. Current Drug Abuse Reviews, 4, 228–240.
Fogel, S. M., & Smith, C. T. (2011). The function of the sleep spindle: A physiological index of intelligence and a mechanism for sleep-dependent memory consolidation. Neuroscience and Biobehavioral Reviews, 35, 1154–1165.
Frank, M. G. (2006). The mystery of sleep function: Current perspectives and future directions. Reviews in the Neurosciences, 17, 375–392.
Freeman, M. P., Fava, M., Lake, J., Trivedi, M. H., Wisner, K. L., & Mischoulon, D. (2010). Complementary and alternative medicine in major depressive disorder: The American Psychiatric Association task force report. The Journal of Clinical Psychiatry, 71, 669–681.
Giedke, H., & Schwärzler, F. (2002). Therapeutic use of sleep deprivation in depression. Sleep Medicine Reviews, 6, 361–377.
Gold, D. R., Rogacz, S. R., Bock, N., Tosteson, T. D., Baum, T. M., Speizer, F. M., & Czeisler, C. A. (1992). Rotating shift work, sleep, and accidents related to sleepiness in hospital nurses. American Journal of Public Health, 82, 1011–1014.
Golden, W. L. (2012). Cognitive hypnotherapy for anxiety disorders. The American Journal of Clinical Hypnosis, 54, 263–274.
Gómez, R. L., Bootzin, R. R., & Nadel, L. (2006). Naps promote abstraction in language-learning infants. Psychological Science, 17, 670–674.
Guilleminault, C., Kirisoglu, C., Bao, G., Arias, V., Chan, A., & Li, K. K. (2005). Adult chronic sleepwalking and its treatment based on polysomnography. Brain, 128, 1062–1069.
Gujar, N., Yoo, S., Hu, P., & Walker, M. P. (2011). Sleep deprivation amplifies reactivity of brain reward networks, biasing the appraisal of positive emotional experiences. The Journal of Neuroscience, 31, 4466–4474.
Guldenmund, P., Vanhaudenhuyse, A., Boly, M., Laureys, S., & Soddu, A. (2012). A default mode of brain function in altered states of consciousness. Archives Italiennes de Biologie, 150, 107–121.
Halász, P. (1993). Arousals without awakening—Dynamic aspect of sleep. Physiology and Behavior, 54, 795–802.
Han, F. (2012). Sleepiness that cannot be overcome: Narcolepsy and cataplexy. Respirology, 17, 1157–1165.
Hardeland, R., Pandi-Perumal, S. R., & Cardinali, D. P. (2006). Melatonin. International Journal of Biochemistry & Cell Biology, 38, 313–316.
Haasen, C., & Krausz, M. (2001). Myths versus experience with respect to cocaine and crack: Learning from the US experience. European Addiction Research, 7, 159–160.
Henry, D., & Rosenthal, L. (2013). “Listening for his breath:” The significance of gender and partner reporting on the diagnosis, management, and treatment of obstructive sleep apnea. Social Science & Medicine, 79, 48–56.
Hicks, R. A., Fernandez, C., & Pelligrini, R. J. (2001). The changing sleep habits of university students: An update. Perceptual and Motor Skills, 93, 648.
Hicks, R. A., Johnson, C., & Pelligrini, R. J. (1992). Changes in the self-reported consistency of normal habitual sleep duration of college students (1978 and 1992). Perceptual and Motor Skills, 75, 1168–1170.
Hilgard, E. R., & Hilgard, J. R. (1994). Hypnosis in the Relief of Pain. New York: Brunner/Mazel.
Hishikawa, Y., & Shimizu, T. (1995). Physiology of REM sleep, cataplexy, and sleep paralysis. Advances in Neurology, 67, 245–271.
Herman, A., & Herman, A. P. (2013). Caffeine’s mechanism of action and its cosmetic use. Skin Pharmacology and Physiology, 26, 8–14.
Hobson, J. A. (2009). REM sleep and dreaming: Towards a theory of protoconsciousness. Nature Reviews Neuroscience, 10, 803–814.
Horikawa,T., Tamaki, M., Miyawaki, Y. & Kamitani, Y. (2013). Neural Decoding of Visual Imagery During Sleep. Science, 340(6132), 639–642. doi:10.1126/science.1234330
Hossain, J. L., & Shapiro, C. M. (2002). The prevalence, cost implications, and management of sleep disorders: An overview. Sleep and Breathing, 6, 85–102.
Huang, L. B., Tsai, M. C., Chen, C. Y., & Hsu, S. C. (2013). The effectiveness of light/dark exposure to treat insomnia in female nurses undertaking shift work during the evening/night shift. Journal of Clinical Sleep Medicine, 9, 641–646.
Huber, R., Ghilardi, M. F., Massimini, M., & Tononi, G. (2004). Local sleep and learning. Nature, 430, 78–81.
Jayanthi, L. D., & Ramamoorthy, S. (2005). Regulation of monoamine transporters: Influence of psychostimulants and therapeutic antidepressants. The AAPS Journal, 7, E728–738.
Julien, R. M. (2005). Opioid analgesics. In A primer of drug action: A comprehensive guide to the actions, uses, and side effects of psychoactive drugs (pp. 461–500). Portland, OR: Worth.
Kihlstrom, J. F. (2013). Neuro-hypnotism: Prospects for hypnosis and neuroscience. Cortex, 49, 365–374.
Klein, D. C., Moore, R. Y., & Reppert, S. M. (Eds.). (1991). Suprachiasmatic nucleus: The mind’s clock. New York, NY: Oxford University Press.
Kogan, N. M., & Mechoulam, R. (2007). Cannabinoids in health and disease. Dialogues in Clinical Neuroscience, 9, 413–430.
Kromann, C. B., & Nielson, C. T. (2012). A case of cola dependency in a woman with recurrent depression. BMC Research Notes, 5, 692.
Lang, A. J., Strauss, J. L., Bomeya, J., Bormann, J. E., Hickman, S. D., Good, R. C., & Essex, M. (2012). The theoretical and empirical basis for meditation as an intervention for PTSD. Behavior Modification, 36, 759–786.
LaBerge, S. (1990). Lucid dreaming: Psychophysiological studies of consciousness during REM sleep. In R. R. Bootzen, J. F. Kihlstrom, & D. L. Schacter (Eds.), Sleep and cognition (pp. 109–126). Washington, DC: American Psychological Association.
Lesku, J. A., Roth, T. C., 2nd, Amlaner, C. J., & Lima, S. L. (2006). A phylogenetic analysis of sleep architecture in mammals: The integration of anatomy, physiology, and ecology. The American Naturalist, 168, 441–453.
Levitt, C., Shaw, E., Wong, S., & Kaczorowski, J. (2007). Systematic review of the literature on postpartum care: Effectiveness of interventions for smoking relapse prevention, cessation, and reduction in postpartum women. Birth, 34, 341–347.
Lifshitz, M., Aubert Bonn, N., Fischer, A., Kashem, I. F., & Raz, A. (2013). Using suggestion to modulate automatic processes: From Stroop to McGurk and beyond. Cortex, 49, 463–473.
Luppi, P. H., Clément, O., Sapin, E., Gervasoni, D., Peyron, C., Léger, L., . . . Fort, P. (2011). The neuronal network responsible for paradoxical sleep and its dysfunctions causing narcolepsy and rapid eye movement (REM) behavior disorder. Sleep Medicine Reviews, 15, 153–163.
Mage, D. T., & Donner, M. (2006). Female resistance to hypoxia: Does it explain the sex difference in mortality rates? Journal of Women’s Health, 15, 786–794.
Mahowald, M. W., & Schenck, C. H. (2000). Diagnosis and management of parasomnias. Clinical Cornerstone, 2, 48–54.
Mahowald, M. W., Schenck, C. H., & Cramer Bornemann, M. A. (2005). Sleep-related violence. Current Neurology and Neuroscience Reports, 5, 153–158.
Mayo Clinic. (n.d.). Sleep terrors (night terrors). Retrieved from http://www.mayoclinic.org/diseases-conditions/night-terrors/basics/treatment/con-20032552
Mather, L. E., Rauwendaal, E. R., Moxham-Hall, V. L., & Wodak, A. D. (2013). (Re)introducing medical cannabis. The Medical Journal of Australia, 199, 759–761.
Maxwell, J. C. (2006). Trends in the abuse of prescription drugs. Gulf Coast Addiction Technology Transfer Center. Retrieved from http://asi.nattc.org/userfiles/file/GulfCoast/PrescriptionTrends_Web.pdf
McCarty, D. E. (2010). A case of narcolepsy with strictly unilateral cataplexy. Journal of Clinical Sleep Medicine, 15, 75–76.
McDaid, C., Durée, K. H., Griffin, S. C., Weatherly, H. L., Stradling, J. R., Davies, R. J., . . . Westwood, M. E. (2009). A systematic review of continuous positive airway pressure for obstructive sleep apnoea-hypopnoea syndrome. Sleep Medicine Reviews, 13, 427–436.
McKim, W. A., & Hancock, S. D. (2013). Drugs and behavior: An introduction to behavioral pharmacology, 7th edition. Boston, MA: Pearson.
Mignot, E. J. M. (2012). A practical guide to the therapy of narcolepsy and hypersomnia syndromes. Neurotherapeutics, 9, 739–752.
Miller, N. L., Shattuck, L. G., & Matsangas, P. (2010). Longitudinal study of sleep patterns of United States Military Academy cadets. Sleep, 33, 1623–1631.
Mitchell, E. A. (2009). SIDS: Past, present and future. Acta Paediatrica, 98, 1712–1719.
Montgomery, G. H., Schnur, J. B., & Kravits, K. (2012). Hypnosis for cancer care: Over 200 years young. CA: A Cancer Journal for Clinicians, 63, 31–44.
National Institutes of Health. (n.d.). Information about sleep. Retrieved from http://science.education.nih.gov/supplements/nih3/sleep/guide/info-sleep.htm
National Research Council. (1994). Learning, remembering, believing: Enhancing human performance. Washington, DC: The National Academies Press.
National Sleep Foundation. (n.d.). How much sleep do we really need? Retrieved from http://sleepfoundation.org/how-sleep-works/how-much-sleep-do-we-really-need
Ohayon, M. M. (1997). Prevalence of DSM-IV diagnostic criteria of insomnia: Distinguishing insomnia related to mental disorders from sleep disorders. Journal of Psychiatric Research, 31, 333–346.
Ohayon, M. M. (2002). Epidemiology of insomnia: What we know and what we still need to learn. Sleep Medicine Reviews, 6, 97–111.
Ohayon, M. M., Carskadon, M. A., Guilleminault, C., & Vitiello, M. V. (2004). Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: Developing normative sleep values across the human lifespan. Sleep, 27, 1255–1273.
Ohayon, M. M., & Roth, T. (2002). Prevalence of restless legs syndrome and periodic limb movement disorder in the general population. Journal of Psychosomatic Research, 53, 547–554.
Poe, G. R., Walsh, C. M., & Bjorness, T. E. (2010). Cognitive neuroscience of sleep. Progress in Brain Research, 185, 1–19.
Porkka-Heiskanen, T. (2011). Methylxanthines and sleep. Handbook of Experimental Pharmacology, 200, 331–348.
Presser, H. B. (1995). Job, family, and gender: Determinants of nonstandard work schedules among employed Americans in 1991. Demography, 32, 577–598.
Pressman, M. R. (2007). Disorders of arousal from sleep and violent behavior: The role of physical contact and proximity. Sleep, 30, 1039–1047.
Provini, F., Tinuper, P., Bisulli, F., & Lagaresi, E. (2011). Arousal disorders [Supplemental material]. Sleep Medicine, 12(2 Suppl.), S22–S26.
Rattenborg, N. C., Lesku, J. A., Martinez-Gonzalez, D., & Lima, S. L. (2007). The non-trivial functions of sleep. Sleep Medicine Reviews, 11, 405–409.
Raz, A. (2011). Hypnosis: A twilight zone of the top-down variety: Few have never heard of hypnosis but most know little about the potential of this mind-body regulation technique for advancing science. Trends in Cognitive Sciences, 15, 555–557.
Raz, A., Shapiro, T., Fan, J., & Posner, M. I. (2002). Hypnotic suggestion and the modulation of Stroop interference. Archives of General Psychiatry, 59, 1151–1161.
Reiner, K., Tibi, L., & Lipsitz, J. D. (2013). Do mindfulness-based interventions reduce pain intensity? A critical review of the literature. Pain Medicine, 14, 230–242.
Restless Legs Syndrome Foundation. (n.d.). Restless legs syndrome: Causes, diagnosis, and treatment for the patient living with Restless legs syndrome (RSL). Retrieved from www.rls.org
Rial, R. V., Nicolau, M. C., Gamundí, A., Akaârir, M., Aparicio, S., Garau, C., . . . Esteban, S. (2007). The trivial function of sleep. Sleep Medicine Reviews, 11, 311–325.
Riemann, D., Berger, M., & Volderholzer, U. (2001). Sleep and depression—Results from psychobiological studies: An overview. Biological Psychology, 57, 67–103.
Reinerman, C. (2007, October 14). 5 myths about that demon crack. Washington Post. Retrieved from http://www.washingtonpost.com/wp-dyn/content/article/2007/10/09/AR2007100900751.html
Reissig, C. J., Strain, E. C., & Griffiths, R. R. (2009). Caffeinated energy drinks—A growing problem. Drug and Alcohol Dependence, 99, 1–10.
Robson, P. J. (2014). Therapeutic potential of cannabinoid medicines. Drug Testing and Analysis, 6, 24–30.
Roth, T. (2007). Insomnia: Definition, prevalence, etiology, and consequences [Supplemental material]. Journal of Clinical Sleep Medicine, 3(5 Suppl.), S7–S10.
Rothman, R. B., Blough, B. E., & Baumann, M. H. (2007). Dual dopamine/serotonin releasers as potential medications for stimulant and alcohol addictions. The AAPS Journal, 9, E1–10.
Sánchez-de-la-Torre, M., Campos-Rodriguez, F., & Barbé, F. (2012). Obstructive sleep apnoea and cardiovascular disease. The Lancet Respiratory Medicine, 1, 31–72.
Savard, J., Simard, S., Ivers, H., & Morin, C. M. (2005). Randomized study on the efficacy of cognitive-behavioral therapy for insomnia secondary to breast cancer, part I: Sleep and psychological effects. Journal of Clinical Oncology, 23, 6083–6096.
Schicho, R., & Storr, M. (2014). Cannabis finds its way into treatment of Crohn’s disease. Pharmacology, 93, 1–3.
Shukla, R. K, Crump, J. L., & Chrisco, E. S. (2012). An evolving problem: Methamphetamine production and trafficking in the United States. International Journal of Drug Policy, 23, 426–435.
Siegel, J. M. (2008). Do all animals sleep? Trends in Neuroscience, 31, 208–213.
Siegel, J. M. (2001). The REM sleep-memory consolidation hypothesis. Science, 294, 1058–1063.
Singh, G. K., & Siahpush, M. (2006). Widening socioeconomic inequalities in US life expectancy, 1980–2000. International Journal of Epidemiology, 35, 969–979.
Smedslund, G., Fisher, K. J., Boles, S. M., & Lichtenstein, E. (2004). The effectiveness of workplace smoking cessation programmes: A meta-analysis of recent studies. Tobacco Control, 13, 197–204.
Sofikitis, N., Giotitsas, N., Tsounapi, P., Baltogiannis, D., Giannakis, D., & Pardalidis, N. (2008). Hormonal regulation of spermatogenesis and spermiogenesis. Journal of Steroid Biochemistry and Molecular Biology, 109, 323–330.
Steriade, M., & Amzica, F. (1998). Slow sleep oscillation, rhythmic K-complexes, and their paroxysmal developments [Supplemental material]. Journal of Sleep Research, 7(1 Suppl.), 30–35.
Stickgold, R. (2005). Sleep-dependent memory consolidation. Nature, 437, 1272–1278.
Stone, K. C., Taylor, D. J., McCrae, C. S., Kalsekar, A., & Lichstein, K. L. (2008). Nonrestorative sleep. Sleep Medicine Reviews, 12, 275–288.
Suchecki, D., Tiba, P. A., & Machado, R. B. (2012). REM sleep rebound as an adaptive response to stressful situations. Frontiers in Neuroscience, 3. doi: 10.3389/fneur.2012.00041
Task Force on Sudden Infant Death Syndrome. (2011). SIDS and other sleep-related infant deaths: Expansion of recommendations for a safe infant sleeping environment. Pediatrics, 128, 1030–1039.
Taillard, J., Philip, P., Coste, O., Sagaspe, P., & Bioulac, B. (2003). The circadian and homeostatic modulation of sleep pressure during wakefulness differs between morning and evening chronotypes. Journal of Sleep Research, 12, 275–282.
Thach, B. T. (2005). The role of respiratory control disorders in SIDS. Respiratory Physiology & Neurobiology, 149, 343–353.
U.S. Food and Drug Administration. (2013, October 24). Statement on Proposed Hydrocodone Reclassification from Janet Woodcock, M.D., Director, Center for Drug Evaluation and Research. Retrieved from http://www.fda.gov/drugs/drugsafety/ucm372089.htm
Vogel, G. W. (1975). A review of REM sleep deprivation. Archives of General Psychiatry, 32, 749–761.
Vøllestad, J., Nielsen, M. B., & Nielsen, G. H. (2012). Mindfulness- and acceptance-based interventions for anxiety disorders: A systematic review and meta-analysis. The British Journal of Clinical Psychology, 51, 239–260.
Wagner, U., Gais, S., & Born, J. (2001). Emotional memory formation is enhanced across sleep intervals with high amounts of rapid eye movement sleep. Learning & Memory, 8, 112–119.
Wagner, U., Gais, S., Haider, H., Verleger, R., & Born, J. (2004). Sleep improves insight. Nature, 427, 352–355.
Walker, M. P. (2009). The role of sleep in cognition and emotion. Annals of the New York Academy of Sciences, 1156, 168–197.
Wark, D. M. (2011). Traditional and alert hypnosis for education: A literature review. The American Journal of Clinical Hypnosis, 54(2), 96–106.
Waterhouse. J., Fukuda, Y., & Morita, T. (2012). Daily rhythms of the sleep-wake cycle [Special issue]. Journal of Physiological Anthropology, 31(5). doi:10.1186/1880-6805-31-5
Welsh, D. K. Takahashi, J. S., & Kay, S. A. (2010). Suprachiasmatic nucleus: Cell autonomy and network properties. Annual Review of Physiology, 72, 551–577.
West, S., Boughton, M., & Byrnes, M. (2009). Juggling multiple temporalities: The shift work story of mid-life nurses. Journal of Nursing Management, 17, 110–119.
White, D. P. (2005). Pathogenesis of obstructive and central sleep apnea. American Journal of Respiratory and Critical Care Medicine, 172, 1363–1370.
Williams, J., Roth, A., Vatthauer, K., & McCrae, C. S. (2013). Cognitive behavioral treatment of insomnia. Chest, 143, 554–565.
Williamson, A. M., & Feyer, A. M. (2000). Moderate sleep deprivation produces impairments in cognitive and motor performance equivalent to legally prescribed levels of alcohol intoxication. Occupational and Environmental Medicine, 57, 649–655.
Wolt, B. J., Ganetsky, M., & Babu, K. M. (2012). Toxicity of energy drinks. Current Opinion in Pediatrics, 24, 243–251.
Zangini, S., Calandra-Buonaura, G., Grimaldi, D., & Cortelli, P. (2011). REM behaviour disorder and neurodegenerative diseases [Supplemental material]. Sleep Medicine, 12(2 Suppl.), S54–S58.
Zeidan, F., Grant, J. A., Brown, C. A., McHaffie, J. G., & Coghill, R. C. (2012). Mindfulness meditation-related pain relief: Evidence for unique brain mechanisms in the regulation of pain. Neuroscience Letters, 520, 165–173.
Licenses and Attributions (Click to expand)
CC licensed content, Original
- Modification, adaptation, and original content. Provided by: Lumen Learning. License: CC BY: Attribution
CC licensed content, Shared previously
- Introduction to States of Consciousness. Authored by: OpenStax College. Located at: https://openstax.org/books/psychology-2e/pages/4-introduction. License: CC BY: Attribution. License Terms: Download for free at https://openstax.org/books/psychology-2e/pages/1-introduction.
- What is Consciousness?. Authored by: OpenStax College. Located at: https://openstax.org/books/psychology-2e/pages/4-1-what-is-consciousness. License: CC BY: Attribution. License Terms: Download for free at https://openstax.org/books/psychology-2e/pages/1-introduction
- Reprogramming Our Circadian Rhythms for the Modern World. Authored by: Big Think. Located at: https://www.youtube.com/watch?v=rtCQ9jzC-Ek. License: Other. License Terms: Standard YouTube License
- Circadian rhythms. Provided by: National Institute of General Medical Science. Located at: https://www.nigms.nih.gov/education/pages/factsheet_circadianrhythms.aspx. License: Public Domain: No Known Copyright
- Zeitgeber. Provided by: Wikipedia. Located at: https://en.wikipedia.org/wiki/Zeitgeber. License: CC BY-SA: Attribution-ShareAlike
- Chronotype. Provided by: Wikipedia. Located at: https://en.wikipedia.org/wiki/Chronotype. License: CC BY-SA: Attribution-ShareAlike
- Visual pathways image. Provided by: Wikimedia. Located at: https://commons.wikimedia.org/wiki/File:Human_visual_pathway.svg. License: CC BY-SA: Attribution-ShareAlike
- College student in the park. Authored by: CollegeDegrees360. Located at: https://www.flickr.com/photos/83633410@N07/7658074952. License: CC BY-SA: Attribution-ShareAlike
- TN Blindsight. Authored by: CANlabTilburg. Located at: https://www.youtube.com/watch?v=ACkxe_5Ubq8. License: Other. License Terms: Standard YouTube License
- TMS image. Authored by: Losey DM, Stocco A, Abernethy JA and Rao RPN . Located at: https://www.frontiersin.org/articles/10.3389/frobt.2016.00072/full. Project: Navigating a 2D Virtual World Using Direct Brain Stimulation.. License: CC BY: Attribution
- Image, neuro-ms. Authored by: Baburov. Provided by: Wikimedia. Located at: https://commons.wikimedia.org/wiki/File:Neuro-ms.png. License: CC BY-SA: Attribution-ShareAlike
Public domain content
- Authored by: Robert Fludd. Provided by: Wikipedia. Located at: https://en.wikipedia.org/wiki/Consciousness#/media/File:RobertFuddBewusstsein17Jh.png. License: Public Domain: No Known Copyright
All rights reserved content
- Seeing Beyond the Visual Cortex – Science Nation. Authored by: National Science Foundation. Located at: https://www.youtube.com/watch?v=_Y4KsUqmuUw. License: Other. License Terms: Standard YouTube License
- Blindsight experiment – 1989. Authored by: Conrad Weiskrantz. Located at: https://www.youtube.com/watch?time_continue=50&v=wDt_Txi7pC0. License: Other. License Terms: Standard YouTube License
- Source: http://marketingland.com/wp-content/ml-loads/2014/09/iceberg-ss-1920.jpg ↵
- A recent literature review of evidence for the existence of the pathways to the cerebral cortex: Rabbo, F. A., Koch, G., Lefevre, C., & Seizeur, R. (2015). Direct geniculo-extrastriate pathways: A review of the literature. Surgical and Radiologic Anatomy, 37(8), 891-899. ↵
- See Consciousness Lost and Found: A Neuropsychological Exploration by Larry Weiskrantz (1997, Oxford University Press). Dr. Weiskrantz is one of the scientists who first described blindsight and studied people with the condition. ↵
- The physics of electromagnetism is fascinating, but we will spare you the details here. You may have studied it in some other class, and there are many readable online sources (e.g., Wikipedia). TMS is a great example of the convergence of technology and psychology that is the basis of modern neuroscience. ↵
awareness of internal and external stimuli
state marked by relatively low levels of physical activity and reduced sensory awareness that is distinct from periods of rest that occur during wakefulness
characterized by high levels of sensory awareness, thought, and behavior
internal cycle of biological activity
biological rhythm that occurs over approximately 24 hours
innate timing device controlled by the suprachiasmatic nucleus
tendency to maintain a balance, or optimal level, within a biological system
area of the hypothalamus in which the body’s biological clock is located
hormone secreted by the endocrine gland that serves as an important regulator of the sleep-wake cycle
endocrine structure located inside the brain that releases melatonin
individual differences in circadian patterns of activity indicating a propensity to sleep at a certain time
brain’s control of switching between sleep and wakefulness as well as coordinating this cycle with the outside world
collection of symptoms brought on by travel from one time zone to another that results from the mismatch between our internal circadian cycles and our environment
a consistent difficulty in falling or staying asleep
work schedule that changes from early to late on a daily or weekly basis
result of insufficient sleep on a chronic basis


