Chapter 5: Sensation and Perception-Part 1
5.4 Other Senses
Learning Objectives
- Summarize the chemical process of taste and smell
- Explain the receptors that respond to touch
- Explain the importance of pain and give examples of how expectations and context affect pain and touch experiences.
- Describe the basic functions of the vestibular, proprioceptive, and kinesthetic sensory systems
- As mentioned earlier, a food’s flavor represents an interaction of both gustatory and olfactory information. Think about the last time you were seriously congested due to a cold or the flu. What changes did you notice in the flavors of the foods that you ate during this time?
Introduction to Other Senses
What you’ll learn to do: describe the basic anatomy and functions of taste, smell, touch, pain, and the vestibular sense
Vision and hearing have received an incredible amount of attention from researchers over the years. While there is still much to be learned about how these sensory systems work, we have a much better understanding of them than of our other sensory modalities. In this section, we will explore our chemical senses (taste and smell) and our body senses (touch, temperature, pain, balance, and body position).
Taste and Smell
Chemical Senses
Taste (Gustation)
You have learned since elementary school that there are four basic groupings of taste: sweet, salty, sour, and bitter. Research demonstrates, however, that we have at least six taste groupings. Umami is our fifth taste. Umami is actually a Japanese word that roughly translates to yummy, and it is associated with a taste for monosodium glutamate (Kinnamon & Vandenbeuch, 2009). There is also a growing body of experimental evidence suggesting that we possess a taste for the fatty content of a given food (Mizushige, Inoue, & Fushiki, 2007).
Molecules from the food and beverages we consume dissolve in our saliva and interact with taste receptors on our tongue and in our mouth and throat. Taste buds are formed by groupings of taste receptor cells with hair-like extensions that protrude into the central pore of the taste bud (Figure 1). Taste buds have a life cycle of ten days to two weeks, so even destroying some by burning your tongue won’t have any long-term effect; they just grow right back. Taste molecules bind to receptors on this extension and cause chemical changes within the sensory cell that result in neural impulses being transmitted to the brain via different nerves, depending on where the receptor is located. Taste information is transmitted to the medulla, thalamus, and limbic system, and to the gustatory cortex, which is tucked underneath the overlap between the frontal and temporal lobes (Maffei, Haley, & Fontanini, 2012; Roper, 2013).

Smell (Olfaction)
Olfactory receptor cells are located in a mucous membrane at the top of the nose. Small hair-like extensions from these receptors serve as the sites for odor molecules dissolved in the mucus to interact with chemical receptors located on these extensions (Figure 2). Once an odor molecule has bound a given receptor, chemical changes within the cell result in signals being sent to the olfactory bulb: a bulb-like structure at the tip of the frontal lobe where the olfactory nerves begin. From the olfactory bulb, information is sent to regions of the limbic system and to the primary olfactory cortex, which is located very near the gustatory cortex (Lodovichi & Belluscio, 2012; Spors et al., 2013).

Olfactory receptors are complex proteins called G protein-coupled receptors (GPCRs). These structures are proteins that weave back and forth across the membranes of olfactory cells seven times, forming structures outside the cell that sense odorant molecules and structures inside the cell that activate the neural message ultimately conveyed to the brain by olfactory neurons. The structures that sense odorants can be thought of as tiny binding pockets with sites that respond to active parts of molecules (e.g., carbon chains). There are about 350 functional olfactory genes in humans; each gene expresses a particular kind of olfactory receptor. All olfactory receptors of a given kind project to structures called glomeruli (paired clusters of cells found on both sides of the brain). For a single molecule, the pattern of activation across the glomeruli paints a picture of the chemical structure of the molecule. Thus, the olfactory system can identify a vast array of chemicals present in the environment. Most of the odors we encounter are actually mixtures of chemicals (e.g., bacon odor). The olfactory system creates an image for the mixture and stores it in memory just as it does for the odor of a single molecule (Shepherd, 2005).
There is tremendous variation in the sensitivity of the olfactory systems of different species. We often think of dogs as having far superior olfactory systems than our own, and indeed, dogs can do some remarkable things with their noses. There is some evidence to suggest that dogs can “smell” dangerous drops in blood glucose levels as well as cancerous tumors (Wells, 2010). Dogs’ extraordinary olfactory abilities may be due to the increased number of functional genes for olfactory receptors (between 800 and 1200), compared to the fewer than 400 observed in humans and other primates (Niimura & Nei, 2007).
Many species respond to chemical messages, known as pheromones, sent by another individual (Wysocki & Preti, 2004). Pheromonal communication often involves providing information about the reproductive status of a potential mate. So, for example, when a female rat is ready to mate, she secretes pheromonal signals that draw attention from nearby male rats. Pheromonal activation is actually an important component in eliciting sexual behavior in the male rat (Furlow, 1996, 2012; Purvis & Haynes, 1972; Sachs, 1997). There has also been a good deal of research (and controversy) about pheromones in humans (Comfort, 1971; Russell, 1976; Wolfgang-Kimball, 1992; Weller, 1998).
Touch and Pain
Touch, Thermoception, and Noiception

The skin can convey many sensations, such as the biting cold of a wind, the comfortable pressure of a hand holding yours, or the irritating itch from a woolen scarf. The different types of information activate specific receptors that convert the stimulation of the skin to electrical nerve impulses, a process called transduction. There are three main groups of receptors in our skin: mechanoreceptors, responding to mechanical stimuli, such as stroking, stretching, or vibration of the skin; thermoreceptors, responding to cold or hot temperatures; and chemoreceptors, responding to certain types of chemicals either applied externally or released within the skin (such as histamine from an inflammation). For an overview of the different receptor types and their properties, see Table 1. The experience of pain usually starts with activation of nociceptors—receptors that fire specifically to potentially tissue-damaging stimuli. Most of the nociceptors are subtypes of either chemoreceptors or mechanoreceptors. When tissue is damaged or inflamed, certain chemical substances are released from the cells, and these substances activate the chemosensitive nociceptors. Mechanoreceptive nociceptors have a high threshold for activation—they respond to mechanical stimulation that is so intense it might damage the tissue. Sensory information collected from the receptors and free nerve endings travels up the spinal cord and is transmitted to regions of the medulla, thalamus, and ultimately to somatosensory cortex, which is located in the postcentral gyrus of the parietal lobe.
| Identity of receptor | Size of receptor* | Type of skin where found | Speed of adaptation* | Adequate stimulus* |
| Merkel’s disks | small, sharp borders | glabrous* | slow | pressure |
| Meissner’s corpusles | small, sharp borders | glabrous | rapid | indentation |
| Ruffini corpuscles | large, diffuse borders | hairy + glabrous | slow | stretching |
| Pacinian corpuscles | large, diffuse borders | hairy + glabrous | rapid | vibration |
| *Terms:
Adequate stimulus-the type of stimulus that the receptor is specialized to receive and respond to. Glabrous skin-the hairless skin found on our palms and the soles of our feet. This skin has a higher density of receptors of a more complex range, which reflects the fact that we use these areas of our body to actively explore our surroundings and to discriminate tactile properties of objects we’re interacting with. Low-threshold mechanoreceptors-mechanoreceptors that respond to stimulus that is so light it doesn’t threaten to damage the tissue around it. high-threshold mechanoreceptors respond to stimulation of higher intensity, and are a type of nociceptor. Receptive field-the space of skin or tissue in which stimulation will elicit a response in the receptor. Smaller receptive fields make the receptor more sensitive to details. Speed adaptation-slowly adapting mechanoreceptors continue to fire action potentials during sustained stimulation. Rapidly adapting mechanoreceptors continue to fire action potentials in response to stimulus onset and offset (i.e. to stimuli changes), and help detect stimulus movement on the skin. |
||||
Pain Perception
Life Without Pain?
Imagine a life free of pain. How would it be—calm, fearless, serene? Would you feel invulnerable, invincible? Getting rid of pain is a popular quest—a quick search for “pain-free life” on Google returns well over 4 million hits—including links to various bestselling self-help guides promising a pain-free life in only 7 steps, 6 weeks, or 3 minutes. Pain management is a billion-dollar market, and involves much more than just pharmaceuticals. Surely a life with no pain would be a better one?
Well, consider one of the “lucky few”: 12-year-old “Thomas” has never felt deep pain. Not even when a fracture made him walk around with one leg shorter than the other, so that the bones of his healthy leg were slowly crushed to destruction underneath the knee joint. For Thomas and other members of a large Swedish family, life without pain is a harsh reality because of a mutated gene that affects the growth of the nerves conducting deep pain. Most of those affected suffer from joint damage and frequent fractures to bones in their feet and hands; some end up in wheelchairs even before they reach puberty (Minde et al., 2004). It turns out pain—generally—serves us well.
Living without a sense of touch sounds less attractive than being free of pain—touch is a source of pleasure and essential to how we feel. Losing the sense of touch has severe implications—something patient G. L. experienced when an antibiotics treatment damaged the type of nerves that signal touch from her skin and the position of her joints and muscles. She reported feeling like she’d lost her physical self from her nose down, making her “disembodied”—like she no longer had any connection to the body attached to her head. If she didn’t look at her arms and legs they could just “wander off” without her knowing—initially she was unable to walk, and even after she relearned this skill she was so dependent on her visual attention that closing her eyes would cause her to land in a hopeless heap on the floor. Only light caresses like those from her children’s hands can make her feel she has a body, but even these sensations remain vague and elusive (Olausson et al., 2002; Sacks, 1985).
Pain is an unpleasant experience that involves both physical and psychological components. Feeling pain is quite adaptive because it makes us aware of an injury, and it motivates us to remove ourselves from the cause of that injury. In addition, pain also makes us less likely to suffer additional injury because we will be gentler with our injured body parts.
Generally speaking, pain can be considered to be neuropathic or inflammatory in nature. Pain that signals some type of tissue damage is known as inflammatory pain. In some situations, pain results from damage to neurons of either the peripheral or central nervous system. As a result, pain signals that are sent to the brain get exaggerated. This type of pain is known as neuropathic pain. Multiple treatment options for pain relief range from relaxation therapy to the use of analgesic medications to deep brain stimulation. The most effective treatment option for a given individual will depend on a number of considerations, including the severity and persistence of the pain and any medical/psychological conditions.
Some individuals are born without the ability to feel pain. This very rare genetic disorder is known as congenital insensitivity to pain (or congenital analgesia). While those with congenital analgesia can detect differences in temperature and pressure, they cannot experience pain. As a result, they often suffer significant injuries. Young children have serious mouth and tongue injuries because they have bitten themselves repeatedly. Not surprisingly, individuals suffering from this disorder have much shorter life expectancies due to their injuries and secondary infections of injured sites (U.S. National Library of Medicine, 2013).
Link to Learning
Watch this video of a girl who feels no pain to learn more about congenital insensitivity to pain.
Action Potentials in the Receptor Cells Travel as Nerve Impulses with Different Speeds
When you step on a pin, this activates a host of mechanoreceptors, many of which are nociceptors. You may have noticed that the sensation changes over time. First you feel a sharp stab that propels you to remove your foot, and only then you feel a wave of more aching pain. The sharp stab is signaled via fast-conducting A-fibers, which project to the somatosensory cortex. This part of the cortex is somatotopically organized—that is, the sensory signals are represented according to where in the body they stem from (see homunculus illustration, Figure 2). The unpleasant ache you feel after the sharp pin stab is a separate, simultaneous signal sent from the nociceptors in your foot via thin C-pain or Aδ-fibers to the insular cortex and other brain regions involved in processing of emotion and interoception (see Figure 3 for a schematic representation of this pathway). The experience of stepping on a pin is, in other words, composed by two separate signals: one discriminatory signal allowing us to localize the touch stimulus and distinguish whether it’s a blunt or a sharp stab; and one affective signal that lets us know that stepping on the pin is bad. It is common to divide pain into sensory–discriminatory and affective–motivational aspects (Auvray, Myin, & Spence, 2010). This distinction corresponds, at least partly, to how this information travels from the peripheral to the central nervous system and how it is processed in the brain (Price, 2000).

Pain Is Necessary for Survival, but Our Brain Can Stop It if It Needs To
In April 2003, the climber Aron Ralston found himself at the floor of Blue John Canyon in Utah, forced to make an appalling choice: face a slow but certain death—or amputate his right arm. Five days earlier he fell down the canyon—since then he had been stuck with his right arm trapped between an 800-lb boulder and the steep sandstone wall. Weak from lack of food and water and close to giving up, it occurred to him like an epiphany that if he broke the two bones in his forearm he could manage to cut off the rest with his pocket knife. The thought of freeing himself and surviving made him so exited he spent the next 40 minutes completely engrossed in the task: first snapping his bones using his body as a lever, then sticking his fingers into the arm, pinching bundles of muscle fibers and severing them one by one, before cutting the blue arteries and the pale “noodle-like” nerves. The pain was unimportant. Only cutting through the thick white main nerve made him stop for a minute—the flood of pain, he describes, was like thrusting his entire arm “into a cauldron of magma.” Finally free, he rappelled down a cliff and walked another 7 miles until he was rescued by some hikers (Ralston, 2010).
How is it possible to do something so excruciatingly painful to yourself, as Aron Ralston did, and still manage to walk, talk, and think rationally afterwards? The answer lies within the brain, where signals from the body are interpreted. When we perceive somatosensory and nociceptive signals from the body, the experience is highly subjective and malleable by motivation, attention, emotion, and context.
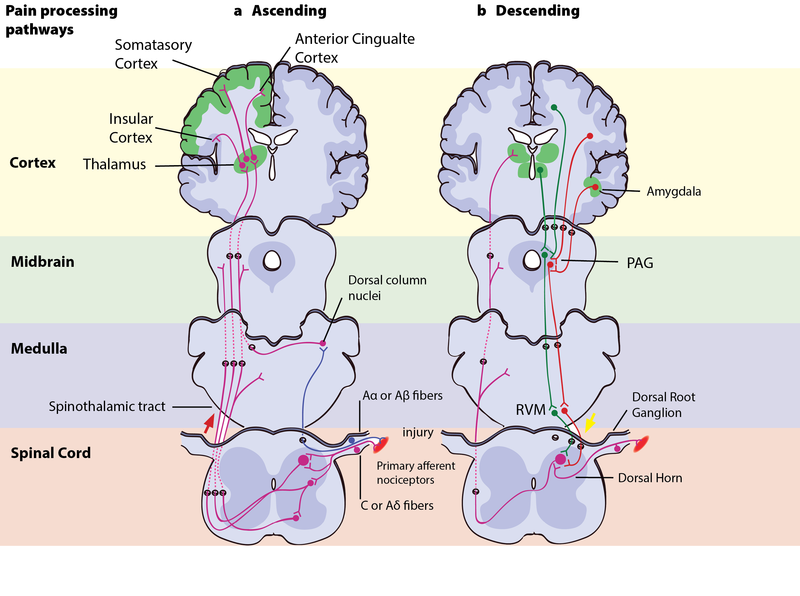
Motivation–Decision Model and Descending Modulation of Pain
According to the motivation–decision model, the brain automatically and continuously evaluates the pros and cons of any situation—weighing impending threats and available rewards (Fields, 2004, 2006). Anything more important for survival than avoiding the pain activates the brain’s descending pain modulatory system—a top-down system involving several parts of the brain and brainstem, which inhibits nociceptive signaling so that the more important actions can be attended to.
In Aron’s extreme case, his actions were likely based on such an unconscious decision process—taking into account his homeostatic state (his hunger, thirst, the inflammation and decay of his crushed hand slowly affecting the rest of his body), the sensory input available (the sweet smell of his dissolving skin, the silence around him indicating his solitude), and his knowledge about the threats facing him (death, or excruciating pain that won’t kill him) versus the potential rewards (survival, seeing his family again). Aron’s story illustrates the evolutionary advantage to being able to shut off pain: The descending pain modulatory system allows us to go through with potentially life-saving actions.
However, when one has reached safety or obtained the reward, healing is more important. The very same descending system can then “crank up” nociception from the body to promote healing and motivate us to avoid potentially painful actions. To facilitate or inhibit nociceptive signals from the body, the descending pain modulatory system uses a set of ON- or OFF-cells in the brainstem, which regulates how much of the nociceptive signal reaches the brain. The descending system is dependent on opioid signaling, and analgesics like morphine relieve pain via this circuit (Petrovic, Kalso, Petersson, & Ingvar, 2002).
Analgesic Power of Reward
Thinking about the good things, like his loved ones and the life ahead of him, was probably pivotal to Aron’s survival. The promise of a reward can be enough to relieve pain. Expecting pain relief (getting less pain is often the best possible outcome if you’re in pain, i.e., it is a reward) from a medical treatment contributes to the placebo effect—where pain relief is due at least partly to your brain’s descending modulation circuit, and such relief depends on the brain’s own opioid system (Eippert et al., 2009; Eippert, Finsterbusch, Bingel, & Buchel, 2009; Levine, Gordon, & Fields, 1978). Eating tasty food, listening to good music, or feeling pleasant touch on your skin also decreases pain in both animals and humans, presumably through the same mechanism in the brain (Leknes & Tracey, 2008).
In a now classic experiment, Dum and Herz (1984) either fed rats normal rat food or let them feast on highly rewarding chocolate-covered candy (rats love sweets) while standing on a metal plate until they learned exactly what to expect when placed there. When the plate was heated up to a noxious/painful level, the rats that expected candy endured the temperature for twice as long as the rats expecting normal chow. Moreover, this effect was completely abolished when the rats’ opioid (endorphin) system was blocked with a drug, indicating that the analgesic effect of reward anticipation was caused by endorphin release.
For Aron the climber, both the stress from knowing that death was impending and the anticipation of the reward it would be to survive probably flooded his brain with endorphins, contributing to the wave of excitement and euphoria he experienced while he carried out the amputation “like a five-year-old unleashed on his Christmas presents” (Ralston, 2010). This altered his experience of the pain from the extreme tissue damage he was causing and enabled him to focus on freeing himself. Our brain, it turns out, can modulate the perception of how unpleasant pain is, while still retaining the ability to experience the intensity of the sensation (Rainville, Duncan, Price, Carrier, & Bushnell, 1997; Rainville, Feine, Bushnell, & Duncan, 1992). Social rewards, like holding the hand of your boyfriend or girlfriend, have pain-reducing effects. Even looking at a picture of him/her can have similar effects—in fact, seeing a picture of a person we feel close to not only reduces subjective pain ratings, but also the activity in pain-related brain areas (Eisenberger et al., 2011). The most common things to do when wanting to help someone through a painful experience—being present and holding the person’s hand—thus seems to have a measurably positive effect.
Power of the Mind
The context of pain and touch has a great impact on how we interpret it. Just imagine how different it would feel to Aron if someone amputated his hand against his will and for no discernible reason. Prolonged pain from injuries can be easier to bear if the incident causing them provides a positive context—like a war wound that testifies to a soldier’s courage and commitment—or phantom pain from a hand that was cut off to enable life to carry on.
The relative meaning of pain is illustrated by a recent experiment, where the same moderately painful heat was administered to participants in two different contexts—one control context where the alternative was a non-painful heat; and another where the alternative was an intensely painful heat. In the control context, where the moderate heat was the least preferable outcome, it was (unsurprisingly) rated as painful. In the other context it was the best possible outcome, and here the exact same moderately painful heat was actually rated as pleasant—because it meant the intensely painful heat had been avoided. This somewhat surprising change in perception—where pain becomes pleasant because it represents relief from something worse—highlights the importance of the meaning individuals ascribe to their pain, which can have decisive effects in pain treatment (Leknes et al., 2013). In the case of touch, knowing who or what is stroking your skin can make all the difference—try thinking about slugs the next time someone strokes your skin if you want an illustration of this point.
Pain and pleasure not only share modulatory systems—another common attribute is that we don’t need to be on the receiving end of it ourselves in order to experience it. How did you feel when you read about Aron cutting through his own tissue, or “Thomas” destroying his own bones unknowingly? Did you cringe? It’s quite likely that some of your brain areas processing affective aspects of pain were active even though the nociceptors in your skin and deep tissue were not firing. Pain can be experienced vicariously, as can itch, pleasurable touch, and other sensations. Tania Singer and her colleagues found in an fMRI study that some of the same brain areas that were active when participants felt pain on their own skin (anterior cingulate and insula) were also active when they were given a signal that a loved one was feeling the pain. Those who were most “empathetic” also showed the largest brain responses (Singer et al., 2004). A similar effect has been found for pleasurable touch: The posterior insula of participants watching videos of someone else’s arm being gently stroked shows the same activation as if they were receiving the touch themselves (Morrison, Bjornsdotter, & Olausson, 2011).
The Vestibular Sense
The Vestibular Sense, Proprioception, and Kinesthesia
The vestibular sense contributes to our ability to maintain balance and body posture. As Figure 1 shows, the major sensory organs (utricle, saccule, and the three semicircular canals) of this system are located next to the cochlea in the inner ear. The vestibular organs are fluid-filled and have hair cells, similar to the ones found in the auditory system, which respond to movement of the head and gravitational forces. When these hair cells are stimulated, they send signals to the brain via the vestibular nerve. Although we may not be consciously aware of our vestibular system’s sensory information under normal circumstances, its importance is apparent when we experience motion sickness and/or dizziness related to infections of the inner ear (Khan & Chang, 2013).

In addition to maintaining balance, the vestibular system collects information critical for controlling movement and the reflexes that move various parts of our bodies to compensate for changes in body position. Therefore, both proprioception (perception of body position) and kinesthesia (perception of the body’s movement through space) interact with information provided by the vestibular system.
These sensory systems also gather information from receptors that respond to stretch and tension in muscles, joints, skin, and tendons (Lackner & DiZio, 2005; Proske, 2006; Proske & Gandevia, 2012). Proprioceptive and kinesthetic information travels to the brain via the spinal column. Several cortical regions in addition to the cerebellum receive information from and send information to the sensory organs of the proprioceptive and kinesthetic systems.
| Definition | Application | |
| Vestibular Sense | Sensory system that contributes to balance and the sense of spatial orientation. | You have an ear infection and frequently feel dizzy. Or if you were to experience vertigo, you might feel like your entire body was spinning in space and be unable to walk. |
| Proprioception | The sense of the position of parts of the body, relative to other neighboring parts of the body. Focuses on the body’s cognitive awareness of movement. | You step off a curb and know where to put your foot. You push an elevator button and control how hard you have to press down with your fingers. |
| Kinesthesia | Awareness of the position and movement of the parts of the body using sensory organs in joints and muscles. Kinesthesia is a key component in muscle memory and hand-eye coordination. It is more behavioral than proprioception. | You are aware of your arm movement while swinging a golf club. Focuses on the body’s movements and not on equilibrium or balance. |
Watch It
Review the things you learned about the senses in the following CrashCourse video.
You can view the transcript for “Homunculus: Crash Course Psychology #6” here (opens in new window).
Licenses and Attributions (Click to expand)
CC licensed content, Original
- The Other Senses. Authored by: OpenStax College. Located at: https://openstax.org/books/psychology-2e/pages/5-5-the-other-senses. License: CC BY: Attribution. License Terms: Download for free at https://openstax.org/books/psychology-2e/pages/1-introduction
- Modification, adaptation, and original content. Provided by: Lumen Learning. License: CC BY: Attribution
- Modification and adaptation, addition of Noba Link to Learning and CrashCourse video. Provided by: Lumen Learning. License: CC BY: Attribution
CC licensed content, Shared previously
- Touch photo. Authored by: Wendy Longo. Located at: https://www.google.com/search?q=5+senses&rlz=1C5CHFA_enUS727US727&source=lnms&tbm=isch&sa=X&ved=0ahUKEwjCwNPv1-zRAhUJj1QKHdLfC-EQ_AUICCgB&biw=1255&bih=743#tbs=sur:fc&tbm=isch&q=touch&imgrc=D65TnqDgRi27_M%3A. License: CC BY-ND: Attribution-NoDerivatives
- The Other Senses. Authored by: OpenStax College. Located at: https://openstax.org/books/psychology-2e/pages/5-5-the-other-senses. License: CC BY: Attribution. License Terms: Download for free at https://openstax.org/books/psychology-2e/pages/1-introduction
- Touch and Pain, information on mechanoreceptors through the power of the mind. Authored by: Guro E. Loseth, Dan-Mikael Ellingson, and Siri Leknes . Provided by: University of Oslo, University of Gothenburg. Located at: http://nobaproject.com/modules/touch-and-pain. Project: The Noba Project. License: CC BY-NC-SA: Attribution-NonCommercial-ShareAlike
- Paragraph on olfactory receptors. Authored by: Linda Bartoshuk and Derek Snyder. Provided by: University of Florida. Located at: http://nobaproject.com/modules/taste-and-smell?r=LDIzOTky. Project: The Noba Project. License: CC BY-NC-SA: Attribution-NonCommercial-ShareAlike
- Homunculus – Crash Course Psychology #6. Provided by: CrashCourse. Located at: https://www.youtube.com/watch?v=fxZWtc0mYpQ. License: Other. License Terms: Standard YouTube License
- Additional Sensory Systems, information for chart. Provided by: Boundless. Located at: https://www.boundless.com/psychology/textbooks/boundless-psychology-textbook/sensation-and-perception-5/sensory-processes-38/additional-sensory-systems-166-12701/. Project: Boundless Psychology. License: CC BY-SA: Attribution-ShareAlike
taste for monosodium glutamate
grouping of taste receptor cells with hair-like extensions that protrude into the central pore of the taste bud
sensory cell for the olfactory system
bulb-like structure at the tip of the frontal lobe, where the olfactory nerves begin
chemical message sent by another individual
touch receptor that responds to pressure and lower frequency vibrations
touch receptor that detects transient pressure and higher frequency vibrations
touch receptor that responds to light touch
touch receptor that detects stretch
signal that some type of tissue damage has occurred
pain from damage to neurons of either the peripheral or central nervous system
genetic disorder that results in the inability to experience pain
sensory signal indicating potential harm and maybe pain
contributes to our ability to maintain balance and body posture
perception of body position
perception of the body’s movement through space


